【论著】| 68Ga-DOTA-PDL1P的设计合成及其在黑色素瘤小鼠模型中的应用研究
时间:2023-09-21 11:09:18 热度:37.1℃ 作者:网络
[摘要] 背景与目的:以程序性死亡[蛋白]-1(programmed death-1,PD-1)/程序性死亡[蛋白]配体-1(programmed death ligand-1,PD-L1)抑制剂为代表的免疫治疗药物在临床上获得巨大成功,但整体有效率差异很大。现阶段在肿瘤开始治疗前,检测患者PD-1/PD-L1的表达情况是临床用药的重要依据,但目前检测PD-L1表达的方法需要获取患者的标本,而获取标本存在创伤性。因此,迫切需要开发一种无创性活体内特异性检测PD-L1表达的方法。我们设计合成了一种新型靶向PD-L1多肽的正电子发射断层显像(positron emission tomography,PET)显像剂68Ga-DOTA-PDL1P,本研究通过对68Ga-DOTA-PDL1P的标记率、放射化学纯度及稳定性进行质量控制评估,并在黑色素瘤小鼠模型中进行应用评价,以期获得有转化前景的68Ga-DOTA-PDL1P新型免疫PET探针。方法:利用镓-68(68Ga)标记 DOTA-PDL1P,并对其放射性化学纯度和稳定性进行质量控制评估;使用鼠源性黑色素瘤B16-F10细胞系进行体外细胞摄取及阻断实验以评价其特异靶向性能;采用B16-F10荷瘤小鼠进行生物分布实验分析该PET显像剂在体内的代谢情况;通过PET/CT显像观察68Ga-DOTA-PDL1P 在B16-F10荷瘤小鼠中的肿瘤摄取程度并进行半定量分析肿瘤靶本比(tumor/muscle,T/M);另外对肿瘤样本进行放射自显影进一步分析肿瘤对68Ga-DOTA-PDL1P探针的摄取情况。采用肿瘤细胞阳性比例分数(tumor cell proportion score,TPS)及联合阳性分数(combined positive score,CPS)指标分析免疫组织化学染色结果评价肿瘤组织的PD-L1表达情况。结果:68Ga-DOTA-PDL1P的标记率及放射化学纯度均大于99%,并在磷酸缓冲盐溶液(phosphate-buffered saline,PBS)和胎牛血清(fetal bovine serum,FBS)中温育3 h后仍保持较好的稳定性(放化纯度均在90%以上)。细胞摄取结果示,68Ga-DOTA-PDL1P可被B16-F10细胞特异性摄取,且该特异性摄取能够被PD-L1P多肽竞争结合阻断。生物分布实验结果示68Ga-DOTA-PDL1P主要经泌尿系统排泄。荷瘤小鼠microPET/CT显像结果表明肿瘤组织高度摄取68Ga-DOTA-PDL1P,T/M为6.7。放射自显影结果证实肿瘤68Ga-DOTA-PDL1P高摄取部位与免疫组织化学染色PD-L1表达阳性区域一致,证明68Ga-DOTA-PDL1P能够特异性与PD-L1阳性的肿瘤细胞结合。结论:本研究合成的68Ga-DOTA-PDL1P新型PET显像剂稳定性好,能特异性靶向PD-L1阳性肿瘤细胞,是一种有转化前景的靶向PD-L1蛋白的新型免疫PET探针。
[关键词]细胞程序性死亡-配体1;黑色素瘤;镓放射性同位素;正电子发射断层显像术
[Abstract]Background and purpose: The immunogenic drugs represented by programmed cell death 1 (PD-1) and programmed death ligand-1 (PD-L1) have achieved great success in clinic. However, with the development of clinical application, the overall effective rate varies greatly. At present, detection of the expression of PD-1/PD-L1 before the start of tumor treatment is an important basis for clinical medication. However, the current detection method of PD-L1 expression requires the acquisition of patient specimens, which is traumatic. Therefore, there is an urgent need to develop a noninvasive method to detect PD-L1 expression in vivo. This study innovatively designed and synthesized a new positron emission tomography (PET) imaging agent targeting PD-L1 polypeptide 68Ga-DOTA-PDL1P. In this study, the labeling rate, radiochemical purity and stability of 68Ga-DOTA-PDL1P were evaluated for quality control. And the application was evaluated in the mouse model of melanoma in order to obtain a promising 68Ga-DOTA-PDL1P immune PET probe. Methods: Gallium-68 (68Ga) was used to label DOTA-PDL1P, and its radiochemical purity and stability were evaluated by quality control. To evaluate its specific targeting performance, the cell uptake and blocking experiments were carried out in murine melanoma B16-F10 cells. The biodistribution experiment was performed in B16-F10 tumor-bearing mice to analyze the metabolism of the PET imaging agent in vivo. The Tumor uptake of 68Ga-DOTA-PDL1P in B16-F10 tumor-bearing mice was observed by PET/CT imaging, and the tumor/muscle (T/M) ratio was semi-quantitatively analyzed. In addition, radioautography was performed to further analyze the tumor uptake of 68Ga-DOTA-PDL1P probe. Tumor cell proportion score (TPS) and combined positive score (CPS) were used to analyze the immunohistochemical staining results to evaluate the expression of PD-L1 in tumor tissues. Results: The labeling efficiency and radiochemical purity of 68Ga-DOTA-PDL1P were more than 99%, and the radiochemical purity of 68Ga-DOTA-PDL1P was more than 90% after 3 h of incubation in PBS and fetal bovine serum. Cellular uptake results showed that 68Ga-DOTA-PDL1P could be specifically taken up by B16-F10 cells, and the specific uptake could be blocked by PD-L1P peptide competitive binding. Biodistribution assay showed that 68Ga-DOTA-PDL1P was mainly excreted through the urinary system. The results of microPET/CT imaging of tumor-bearing mice showed a high uptake of 68Ga-DOTA-PDL1P in tumor tissues, and the T/M ratio was 6.7. The autoradiography results confirmed that 68Ga-DOTA-PDL1P high uptake in the tumor was consistent with the PD-L1 positive area of immunohistochemical staining, which proved that 68Ga-DOTA-PDL1P could specifically bind to PD-L1 positive malignant tumor cells. Conclusion: The novel 68Ga-DOTA-PDL1P PET imaging agent synthesized in this study has good stability and can specifically target PD-L1 positive tumor cells, which is a promising new immune PET probe targeting PD-L1 protein.
[Keywords] PD-L1; Malignant melanoma; 68Ga; PET/CT
免疫系统在抑制癌症中起着重要作用。针对程序性死亡[蛋白]-1( programmed death-1,PD-1)/程序性死亡[蛋白]配体-1(programmed death ligand-1,PD-L1)的抗体分别掩盖免疫效应细胞上的PD-1受体和肿瘤细胞上的PD-L1配体,通过阻断PD-1/PD-L1结合增强对肿瘤的免疫应答[1-3]。由于恶性肿瘤存在恶性程度高及复发概率大等因素,除手术治疗外还需联合辅助治疗[4-7]。在临床实践中,部分恶性肿瘤患者对PD-1免疫治疗表现出长期应答[8]。研究[9]表明,抗PD-1疗法在转移性黑色素瘤的治疗中比化疗更有效。肿瘤PD-L1表达是PD-1/PD-L1免疫治疗患者筛选的一个重要依据,目前PD-L1的表达检测仍以传统的免疫组织化学方法为主。然而免疫组织化学方法检测PD-L1在肿瘤中的表达存在一些局限。首先,作为侵入性检查,穿刺活检可能会受到患者各种身体状况的限制。其次,活检只能检测到特定的病变,也许不能充分体现PD-L1表达在所有转移瘤中的异质性,而肿瘤PD-L1表达的全面情况对临床治疗具有指导意义。已有研究[10]指出部分PD-L1阴性的患者对PD-1治疗仍有良好的反应,这可能是因为活检可能没有捕捉到肿瘤上PD-L1的异质性表达。因此,迫切需要开发一种可重复性、特异性的无创检测PD-L1表达的方法。由于正电子发射断层显像(positron emission tomography,PET)/计算机体层成像(computed tomography,CT)成像的独特优势,目前对新型PET探针靶向特异显像的研究[11-12]发展很快,而对新型PET探针PD-L1表达预测的研究[13-16]亦较多,[18F]FPy-WL12能特异性检测肿瘤中PD-L1的表达,但需要进一步优化该示踪剂,以改善其在体内的分布和图像对比度。Chang等[17]报道了一种PD-L1的拮抗剂DPPA-1,是一种特异性靶向PD-L1的小分子直链多肽。本研究在该多肽的基础上设计合成了一种新的靶向PD-L1的放射性分子影像探针,并初步在荷瘤鼠中评估了其检测PD-L1表达的能力。
1 资料和方法
1.1 68Ga-DOTA-PDL1P的放射性标记
10 μg DOTA-PDL1P(PD-L1-targeted peptide)溶于NaAc溶液,加入68GaCl3洗脱液,调节pH至4,将上述混合物于沸水浴中加热10 min。反应后进行取样分析。以ITLC纸色谱为固定相,NH4AC/CH3OH(1︰1)为展开剂,采用放射性薄层色谱(radioactive thin-layer chromatography,Radio-TLC)分析68Ga-DOTA-PDL1P的放射化学纯度。68Ga-DOTA-PDL1P的放射化学纯度也通过分析高效液相色谱(high performance liquid chromatography,HPLC)进行了评估。
1.2 68Ga-DOTA-PDL1P体外稳定性研究
取3.7 mBq 68Ga-DOTA-PDL1P分别加入到磷酸缓冲盐溶液(phosphate-buffered saline,PBS)和胎牛血清(fetal bovine serum,FBS)中,37℃共同温育3 h,温育结束后采用Radio-TLC分析68Ga-DOTA-PDL1P的体外稳定性。
1.3 细胞实验及肿瘤模型的建立
鼠源性黑色素瘤B16-F10细胞系在添加了含10%FBS和谷氨酰胺的DMEM培养基(美国Gibco公司)中培养,培养箱条件设置为37℃、CO2体积分数为5%。完成细胞计数后传至24孔板中,密度为2.0×106~2.5×106个/孔,继续温育24 h。随后在铺有B16-F10细胞的24孔板每孔加入1 mL含1 μCi 68Ga-DOTA-PDL1P的DMEM培养液,把上述24孔板置于37℃、CO2体积分数为5%的培养箱中分别温育1、2及4 h,每个时间点设置6个复孔。在24孔板中的另外6个复孔中加入1 mL的1 μCi 68Ga-DOTA-PDL1P显像剂的同时加入PD-L1P抗体前体并温育2 h。用γ计数器(SN-697,上海核所日环光电仪器有限公司)测定细胞摄取量。
接种肿瘤建立荷瘤鼠模型时,用0.25%的胰蛋白酶消化细胞,然后离心机离心,制成1×106个B16-F10细胞/100 μL的细胞悬液。于每只6~8周龄(18~20 g左右)雌性BALB/c小鼠(购自灵畅生物技术有限公司)右侧腋下皮下注射100 μL上述细胞悬液,在IVC级动物房饲养1周,每2 d使用游标卡尺测量肿瘤直径并记录数据,等到肿瘤直径达到0.8~1.0 cm时,可以进行实验。所有动物实验均通过复旦大学实验动物伦理委员会批准。
1.4 体内PET/CT成像
荷B16-F10瘤鼠经尾静脉注射约7.4 MBq的68Ga-DOTA-PDL1P。注射1 h后,使用Micro-PET/CT(德国Siemens公司)对荷瘤鼠进行静态全身PET/CT成像。重建后的图像数据使用microPET/CT自带重建软件进行处理分析。统计分析和计算靶本比(tumor/muscle,T/M),3D感兴趣区域(regions of interest,ROI)包括每只小鼠的整个肿瘤,在对侧正常肌肉组织区域绘制直径相似的背景ROI。通过软件计算各ROI的放射性浓度(μCi/mm3)。T/M表示肿瘤信号强度,旨在减少小鼠间的差异。
1.5 生物分布、放射自显影及免疫组织化学分析
PET/CT成像后,进行放射自显影和PD-L1免疫组织化学检测。取肿瘤组织,切成两半。将其中一半轻轻压在荧光屏上进行放射自显影,另一半用4%的甲醛溶液固定48 h,石蜡包埋,然后切成4 μm切片进行PD-L1免疫染色。将荷瘤小鼠安乐死进行生物分布测定。处死后立即取心、肺、脾、脑、瘤、骨骼肌、骨、血、肝、肾称重。使用γ计数器测量每个样品的放射性。所有γ计数都根据注入时间进行校正,68Ga-DOTA-PDL1P的摄取以每克组织注射剂量的百分比(%ID/g)表示(x±s)。本研究已获得复旦大学附属肿瘤医院实验动物管理和使用委员会伦理批准。
1.6 统计学处理
所有数据以x±s表示。定量数据正态性检验采用双尾单样本Kolmogorov-Smirnov检验。采用t检验及线性回归分析方法进行统计学分析,P <0.05为差异有统计学意义。数据分析采用SPSS 20.0软件。
2 结 果
2.1 68Ga-DOTA-PDL1P的合成、质量控制和体外稳定性
68Ga-DOTA-PDL1P的结构及合成示意图见图1A。Radio-TLC和HPLC结果均显示放化纯度均高于99%(图1B~C)。因此,68Ga-DOTA-PDL1P可以直接用于进一步的体外和体内研究。体外稳定性实验表明68Ga-DOTA-PDL1P在PBS和FBS中温育3 h后均能保持放化纯度在90%以上,说明68Ga-DOTA-PDL1P具有较好的稳定性。
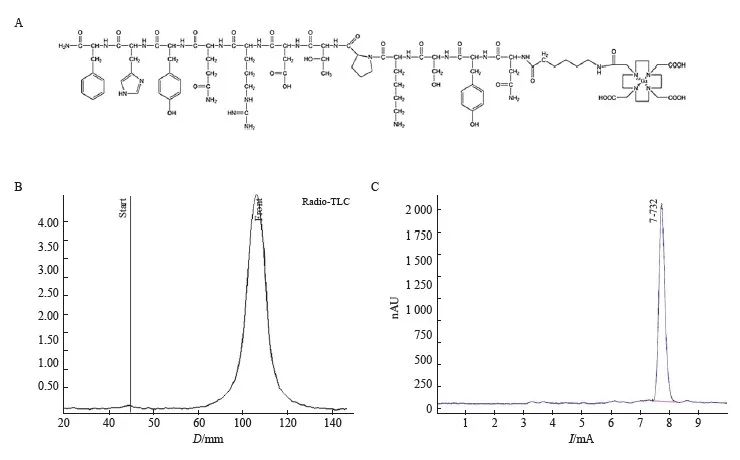
图1 68Ga-DOTA-PDL1P的放射性标记及质量控制
Fig. 1 Radiolabeling and quality control of 68Ga-DOTA-PDL1P
A: Schematic diagram of synthesis; B: Radio-TLC chromatogram; Thin high performance liquid chromatography (HPLC) detection map.
2.2 细胞摄取和体外靶向验证
细胞摄取实验分为实验组(68Ga-DOTA-PDL1P)和2 h阻断对比组(68Ga-DOTA-PDL1P+PD-L1P多肽)。实验组细胞摄取随时间持续增加(P<0.05)。同时,体外靶向结果显示,靶向组与阻断组的细胞摄取量差异有统计学意义(P<0.01,图2)。
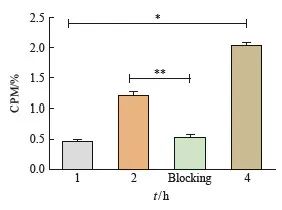
图2 细胞摄取实验
Fig. 2 Cell uptake experiments
The total amount of radioactivity in the four groups was 1 μCi, and B16-F10 cells were incubated for 1, 2 and 4 hours, respectively. Blocking refers to the cell intake of 2 hours targeted blocking group; *:P<0.05, compared with each other; **: P<0.01, compared with each other.
2.3 68Ga-DOTA-PDL1P在B16-F10荷瘤小鼠的生物分布
68Ga-DOTA-PDL1P在B16-F10荷瘤小鼠的生物分布测定结果见图3,68Ga-DOTA-PDL1P在小鼠体内吸收及清除均较快速,其中泌尿系统内放射强度最高,并随时间的延长而浓度下降。
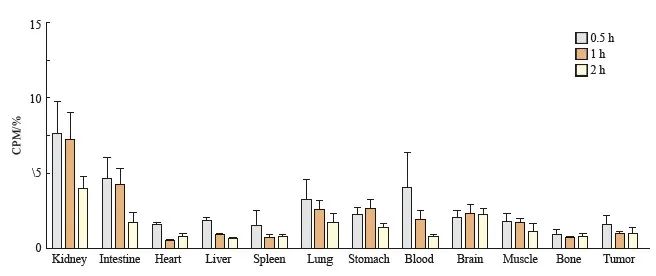
图3 生物分布实验
Fig. 3 Biological distribution experiment
Biodistribution of 68Ga-DOTA-PDL1P at different phases in B16-F10 tumor bearing mice (%ID/g).
2.4 microPET/CT显像
当B16-F10荷瘤小鼠肿瘤大小符合显像要求后,经尾静脉注射68Ga-DOTA-PDL1P后1 h行microPET/CT显像。肿瘤部位放射性摄取明显增高,同时可见血池、胃肠和膀胱部位等器官显影(图4A)。当microPET/CT显像结束后,我们通过重建后的图像数据进行统计分析和计算得到T/M为6.7。
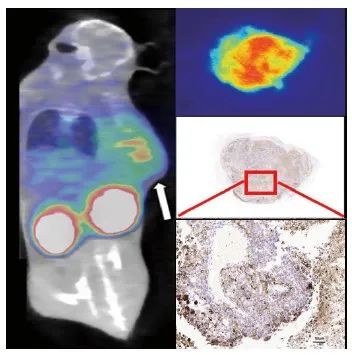
图4 综合评估PD-L1表达情况
Fig. 4 Comprehensive evaluation of PD-L1 expression
A: PET/CT imaging of 68Ga-DOTA-PDL1P in mice bearing B16-F10 tumor; B: Autoradiography of tumor tissue; C-D: Immunohistochemical PD-L1 staining.
2.5 放射自显影及免疫组织化学
荷B16-F10瘤鼠的肿瘤组织放射自显影结果见图4B,肿瘤组织表现为放射性明显摄取;PD-L1免疫组织化学检测结果显示同一肿瘤组织的PD-L1染色呈阳性(图4C),与放射自显影结果相符合。我们采用肿瘤细胞阳性比例分数(tumor cell proportion score,TPS)及联合阳性分数(combined positive score,CPS)指标评价肿瘤组织的PD-L1免疫组织化学检测结果。结果显示,所有组织样本中PD-L1染色TPS及CPS均大于10.0%,其中CPS最低阳性分数为 12.5%,最高为38.3%。此外,进一步分析结果表明PD-L1阳性表达指数包括TPS及CPS均与68Ga-DOTA-PDL1P PET/CT显像代谢参数T/M均呈正相关(β=7.905和9.329,R2=0.950 0和0.937 6, P=0.001 0和0.001 5,图5)。
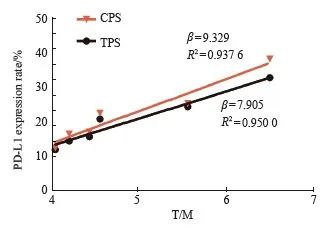
图5 68Ga-DOTA-PDL1P PET/CT显像代谢参T/M与PD-L1表达程度的分析
Fig. 5 Analysis of metabolic parameters T/M and PD-L1
expression in 68Ga-DOTA-PDL1P PET/CT imaging T/M increased with the increase of PD-L1 positive expression index TPS and CPS (β=7.905 and 9.329, R2=0.950 0 and 0.937 6, P=0.001 0 and 0.001 5).
3 讨 论
免疫检查点阻断是近年来恶性肿瘤免疫治疗的重大突破。有研究[2,18- 20]表明,PD-L1在多种恶性肿瘤中高表达,导致肿瘤微环境免疫应答紊乱。有研究[21]报道,抗PD-1和抗PD-L1药物在恶性肿瘤尤其是黑色素瘤中表现出良好的抗肿瘤作用。判断PD-L1在肿瘤中表达情况的传统方法具有不可重复性、有创性等限制因素,而分子影像技术PET/CT利用新型PET分子影像探针可以无创且有效地检测PD-L1表达[13-15,22]。本研究设计的68Ga-DOTA-PDL1P探针正是基于上述临床发展需求而提出的一种精准预测黑色素瘤PD-L1表达情况的方法。
Radio-TLC及HPLC结果均显示,68Ga-DOTA-PDL1P标记率均大于99%,无需纯化可直接用于后续的体内外研究。体外稳定性实验表明68Ga-DOTA-PDL1P具有较好的稳定性。后续细胞摄取实验结果表明,68Ga-DOTA-PDL1P能被B16-F10细胞主动摄取,并且摄取过程能被未标记的PD-L1P多肽竞争性结合阻断,初步证明了B16-F10细胞摄取68Ga-DOTA-PDL1P的特异性。小动物PET/CT显像结果显示荷黑色素瘤小鼠的肿瘤部位的放射性摄取明显增高,结合体外细胞实验及放射自显影结果表明,荷瘤小鼠肿瘤部位PD-L1表达阳性,预示着68Ga-DOTA-PDL1P分子影像探针可以方便而快速地观测黑色素瘤内PD-L1表达情况。免疫组织化学检测结果显示,PD-L1表达率评价指标TPS及CPS均大于10.0%,表明肿瘤组织样本的PD-L1表达呈阳性[23];T/M半定量结果分析进一步证实68Ga-DOTA-PDL1P显像剂可检测肿瘤PD-L1表达情况,且与PD-L1表达率呈正相关。同时膀胱部位可见大量放射性分布,表明该探针通过泌尿系统排泄。
68Ga-DOTA-PDL1P在B16-F10荷瘤小鼠的生物分布结果显示其在体内吸收及清除均较快,其中尿液浓度最高,浓度随时间的延长而下降。这与PET/CT显像结果吻合,再次验证该探针主要由泌尿系统排泄。以上结果表明68Ga-DOTA-PDL1P分子影像探针能精准地检测肿瘤组织中PD-L1的表达情况,并且该探针可以通过泌尿系统安全地排出体外。
综上所述,本研究设计并合成的68Ga-DOTA-PDL1P新型分子影像探针可以无创、有效、动态地检测黑色素瘤中PD-L1表达情况,目前只通过一种PD-L1高表达的B16-F10黑色素瘤细胞系对
68Ga-DOTA-PDL1P探针进行应用研究,后续将通过更多PD-L1高表达的恶性肿瘤验证该探针的临床应用价值。另外,68Ga-DOTA-PDL1P新型分子影像探针目前只能检测肿瘤PD-L1的表达结果,尚缺乏免疫治疗前后的对照研究。未来,我们还将在免疫治疗方面探索更多的靶向探针,并对DOTA-PDL1P的结构进行修饰,改善示踪剂的特性,减少其在非靶器官积累。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1] HERBST R S, SORIA J C, KOWANETZ M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J]. Nature, 2014, 515(7528): 563-567.
[2] ZAK K M, KITEL R, PRZETOCKA S, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1[J]. Structure, 2015, 23(12): 2341-2348.
[3] SAKUISHI K, APETOH L, SULLIVAN J M, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity[J]. J Exp Med, 2010, 207(10): 2187-2194.
[4] SCHADENDORF D, VAN AKKOOI A C J, BERKING C, et al. Melanoma[J]. Lancet, 2018, 392(10151): 971-984.
[5] DIKA, PATRIZI A, LAMBERTINI M, et al. Estrogen receptors and melanoma: a review[J]. Cells, 2019, 8(11): 1463.
[6] MILLER K D, NOGUEIRA L, DEVASIA T, et al. Cancer treatment and survivorship statistics, 2022[J]. CA Cancer J Clin, 2022, 72(5): 409-436.
[7] GERSHENWALD J E, SCOLYER R A, HESS K R, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual[J]. CA Cancer J Clin, 2017, 67(6): 472-492.
[8] EGGERMONT A M M, DUMMER R. The 2017 complete overhaul of adjuvant therapies for high-risk melanoma and its consequences for staging and management of melanoma patients[J]. Eur J Cancer, 2017, 86: 101-105.
[9] HOMET MORENO B, RIBAS A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers[J]. Br J Cancer, 2015, 112(9): 1421-1427.
[10] GARON E B, RIZVI N A, HUI R N, et al. Pembrolizumab for the treatment of non-small cell lung cancer[J]. N Engl J Med, 2015, 372(21): 2018-2028.
[11] VAZ S C, OLIVEIRA F, HERRMANN K, et al. Nuclear medicine and molecular imaging advances in the 21st century[J]. Br J Radiol, 2020, 93(1110): 20200095.
[12] 张 倩, 丁 缙, 任亚楠, 等. 靶向血管紧张素转化酶2新型PET探针的制备及其肿瘤特异性分子显像[J]. 中华核医学与分子影像杂志, 2021, 41(10): 580-584.
ZHANG Q, DING J, REN Y N, et al. Preparation and tumorspecific molecular imaging of a novel PET probe targeting angiotensin converting enzyme 2[J]. Chin J Nucl Med Mol Imaging, 2021, 41(10): 580-584.
[13] CHATTERJEE S, LESNIAK W G, MILLER M S, et al. Rapid PD-L1 detection in tumors with PET using a highly specific peptide[J]. Biochem Biophys Res Commun, 2017, 483(1): 258-263.
[14] DE SILVA R A, KUMAR D, LISOK A, et al. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer[J]. Mol Pharm, 2018, 15(9): 3946-3952.
[15] LESNIAK W G, MEASE R C, CHATTERJEE S, et al. Development of[18F]FPy-WL12 as a PD-L1 specific PET imaging peptide[J] . Mol Imaging ,019, 18 : 1536012119852189.
[16] 赵 亮, 富凯丽, 姚兰琳, 等. 整合素α vβ 3放射性核素靶向治疗联合PD-L1免疫治疗的实验研究[J]. 中华核医学与分子影像杂志, 2020, 40(5): 268-274.
ZHAO L, FU K L, YAO L L, et al. Enhancement of therapeutic efficacy by combination of integrin α vβ 3-targeted radiotherapy and anti-PD-L1 immunotherapy: a preclinical study[J]. Chin J Nucl Med Mol Imaging, 2020, 40(5): 268-274.
[17] CHANG H N, LIU B Y, QI Y K, et al. Blocking of the PD-1/PD-L1 interaction by a D-peptide antagonist for cancer immunotherapy[J]. Angew Chem Int Ed Engl, 2015, 54(40): 11760-11764.
[18] SUN S Y, FEI X C, MAO Y, et al. PD-1( + ) immune cell infiltration inversely correlates with survival of operable breast cancer patients[J]. Cancer Immunol Immunother, 2014, 63(4): 395-406.
[19] AHMADZADEH M, JOHNSON L A, HEEMSKERK B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired[J]. Blood, 2009, 114(8): 1537-1544.
[20] HAWKES E A, GRIGG A, CHONG G. Programmed cell death-1 inhibition in lymphoma[J]. Lancet Oncol, 2015,16(5): e234-e245.
[21] MAHONEY K M, FREEMAN G J, MCDERMOTT D F. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma[J]. Clin Ther, 2015, 37(4): 764-782.
[22] 孙艳莎, 宋少莉. 核素标记的PD-1与PD-L1显像剂在肿瘤诊断中的研究进展[J]. 中华核医学与分子影像杂志, 2019, 39(2): 108-111.
SUN Y S, SONG S L. Research progress of radionuclide labeled imaging agents targeting PD-1 and PD-L1 in the diagnosis of tumors[J]. Chin J Nucl Med Mol Imaging, 2019, 39(2): 108-111.
[23] HUANG Z L, JIN Y, CAI X, et al. Association of the programmed death ligand-1 combined positive score in tumors and clinicopathological features in esophageal cancer[J]. Thorac Cancer, 2022, 13(4): 523-532.


