同济大学陈峰、赵新宇/复旦大学王艳Adv. Sci.:调节铁稳态的仿生支架重塑感染的成骨微环境
时间:2024-10-14 06:03:28 热度:37.1℃ 作者:网络
感染性骨缺损(IBDs)的治疗需要同时消除感染并加速骨再生。IBDs再生的一个阻碍机制是病原体与宿主细胞之间的铁竞争,导致铁缺乏微环境,损害先天免疫反应。该研究提出了一种原位修饰策略,用于打印具有铁稳态调节能力的铁活性多功能支架,用于治疗IBD。在本研究中,我们提出了一种原位修饰策略,用于打印具有调节铁稳态能力的铁活性多功能支架,以治疗IBDs。作为概念验证,我们通过原位生长铁没食子酸盐(FeGA)层,然后将其嵌入聚乳酸-羟基乙酸共聚物(PLGA)基质中,打印了基于PLGA的复合支架,包含FeGA修饰的羟基磷灰石(HA)纳米线(FeGA-HA@PLGA)。FeGA的光热效应赋予了支架出色的抗菌活性。从FeGA-HA@PLGA释放的铁离子有助于恢复铁稳态微环境,从而促进抗炎、血管生成和成骨分化。转录组分析表明,FeGA-HA@PLGA支架通过激活NF-κB、MAPK和PI3K-AKT信号通路,发挥抗炎和促成骨分化的作用。动物实验证实了FeGA-HA@PLGA支架在IBDs骨修复中的卓越性能,表明铁稳态调节治疗在临床应用中具有广阔的前景。

感染性骨缺损(IBD)是骨科和颌面外科的一种严重并发症,通常会导致持续性炎症、骨愈合延迟,有时甚至导致截肢甚至死亡。为了治疗IBD,各种抗菌剂(包括抗生素、贵金属离子和将光转化为热的黑色材料)已被引入骨支架以消除感染。然而,这些抗菌剂由于高剂量毒性和/或生物降解性差,往往引起长期安全问题。此外,这些抗菌剂的生物活性为惰性,这可能会损害支架的骨修复效果。因此,迫切需要具有可生物降解抗菌剂的多功能骨支架,这些抗菌剂具有重建成骨微环境等生物活性功能,但仍然具有挑战性。
在各种生物活性金属离子中,铁基MPNs具有光热抗菌活性和补铁的双重功能。铁在体内处于微妙的平衡状态,它是唯一一种对宿主的营养状况和感染状态都有反应的激素调节微量元素。在感染部位,细菌可以与宿主细胞竞争铁以维持其生存和增殖,从而导致组织铁稳态失衡,从而抑制中性粒细胞的成熟及其免疫防御能力。治疗IBD的一个重要因素是补铁,补铁可以逆转缺铁的微环境,重启先天免疫系统中细胞的免疫防御功能。
在本研究中,提出了一种原位生长策略,以HA和PLGA为基质,制备具有抗菌活性、抗炎和铁稳态调节能力的没食子酸铁(FeGA)金属有机复合物修饰的3D打印铁活性多功能骨支架(方案1)。原位生长策略使用最少量的FeGA将白色HA纳米线变为黑色,从而赋予其优异的光热性能,同时避免潜在的毒性。利用分子动力学(MD)模拟阐明了HA纳米线表面FeGA的层状结构。所获得的由FeGA修饰的HA纳米线(FeGA-HA@PLGA)组成的多功能支架具有设计好的微尺度多孔结构,有利于细胞粘附、迁移和生长,并在其降解时持续释放生物活性铁离子和GA分子。这些释放的铁离子可以逆转缺铁微环境,在感染骨修复的早期阶段重新激活先天免疫防御系统。光热效应通过有效的热模拟增强细菌膜的通透性,使FeGA-HA@PLGA支架具有优异的抗菌性能。释放的GA分子可以清除ROS,而释放的铁离子可以促进巨噬细胞向M2表型转化。GA和铁离子的生物活性有助于将炎症微环境转化为有利于组织再生的微环境。通过协同抗菌和抗炎活性、免疫系统再激活和促成骨分化特性,作者的FeGA-HA@PLGA支架有望对IBD具有良好的治疗效果。
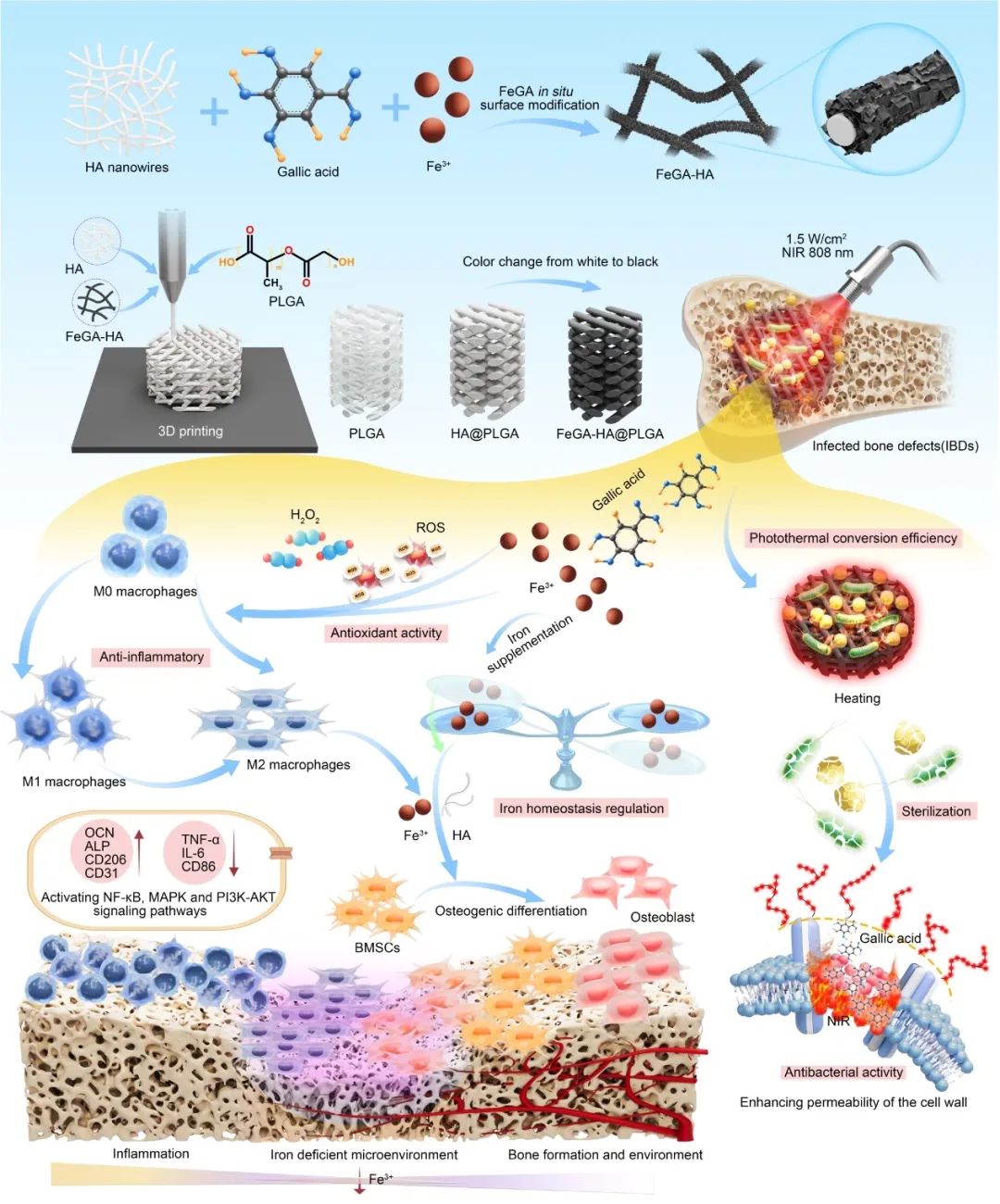
FeGA-HA@PLGA支架治疗IBD的制备及作用机理示意图。
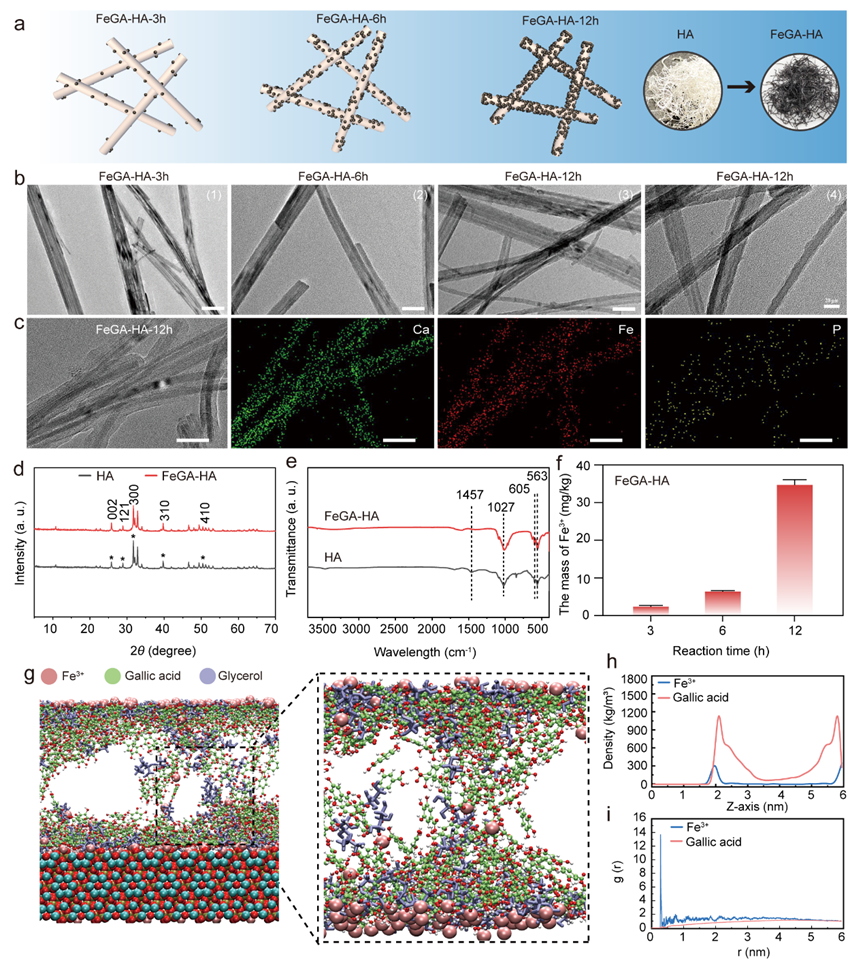
Figure 1. Characterization of the FeGA-HA. (a) Schematic illustration of the formation mechanism of the FeGA-HA samples with increasing reaction time. (b) TEM images of FeGA-HA samples prepared under different reaction times: (1)-(3) Scale bar: 50 nm, (4) Scale bar: 20 nm. (c) TEM image and corresponding elemental mapping images of FeGA-HA-12h, Scale bar: 50 nm. (d) XRD patterns of HA and FeGA-HA. (e) FTIR curves of HA and FeGA-HA. (f) The content of Fe3+ in the FeGA-HA measured by ICP. (g) The MD simulation results show the adsorption structure of FeGA on the HA surface. (h) The relationship between the density of GA and Fe3+ in the system and their distance from the surface of HA. (i) Radial distribution of Fe3+ and GA in the system before and after MD simulation.
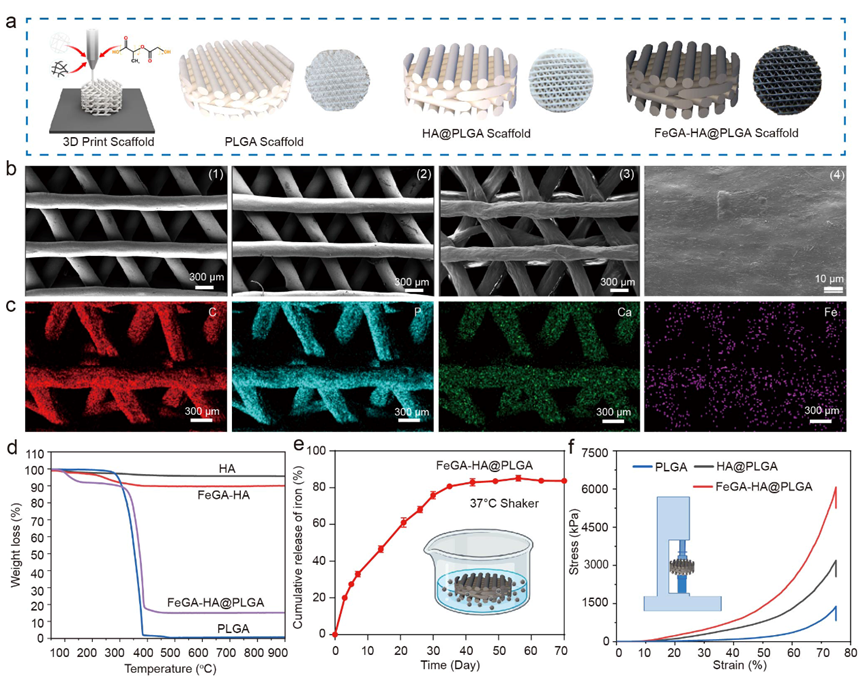
Figure 2. Characterization of FeGA-HA@PLGA. (a) Schematic illustration and digital photos of PLGA, HA@PLGA and FeGA-HA@PLGA scaffolds. (b) SEM images of different scaffolds of PLGA (1), HA@PLGA (2) and FeGA-HA@PLGA (3-4); (1-3) Scale bar: 300 μm. (4) Scale bar: 10 μm. (c) EDS analysis of C, P, Ca, and Fe elements in the FeGA-HA@PLGA scaffolds, Scale bar: 300 μm. (d) The cumulative release of Fe3+ from the FeGA-HA@PLGA scaffolds. (e) The thermal gravimetric analysis of HA, FeGA-HA, PLGA, and FeGA-HA@PLGA. (f) Compressive stress−strain curves of PLGA, FeGA-HA and FeGA-HA@PLGA scaffolds.
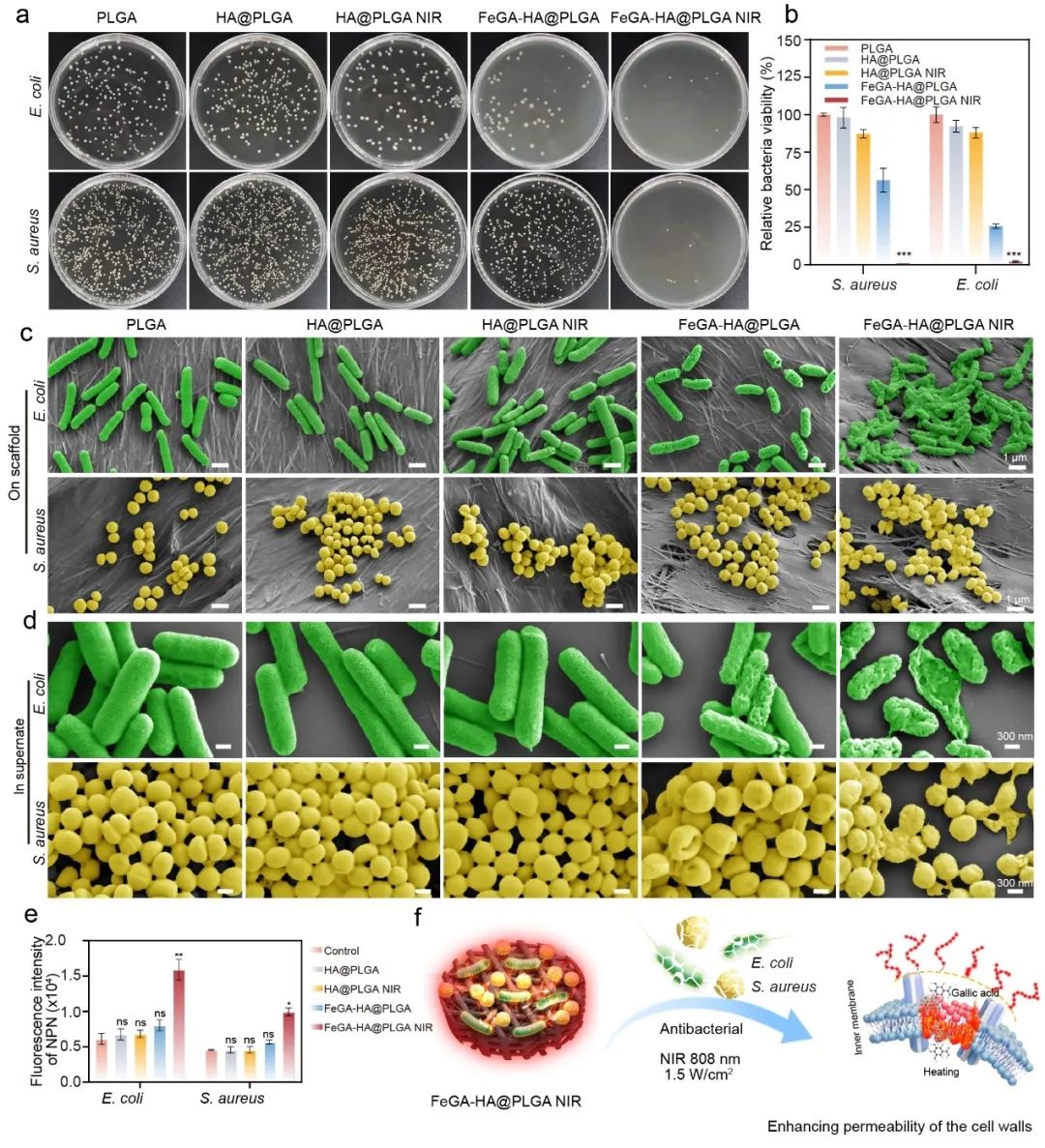
Figure 3. Antibacterial properties of the FeGA-HA@PLGA scaffolds under 808 nm NIR irradiation. (a) Photographs of bacterial colonies of S. aureus and E. coli treated by. (b) The relative viability of bacteria treated with different groups. (c-d) SEM images of S. aureus and E. coli on scaffolds or in supernate after treatment with different groups. (e) Influences of different scaffolds of bacterial membrane permeability. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (f) Schematic diagram of antibacterial mechanism of the FeGA-HA@PLGA scaffolds under NIR irradiation.
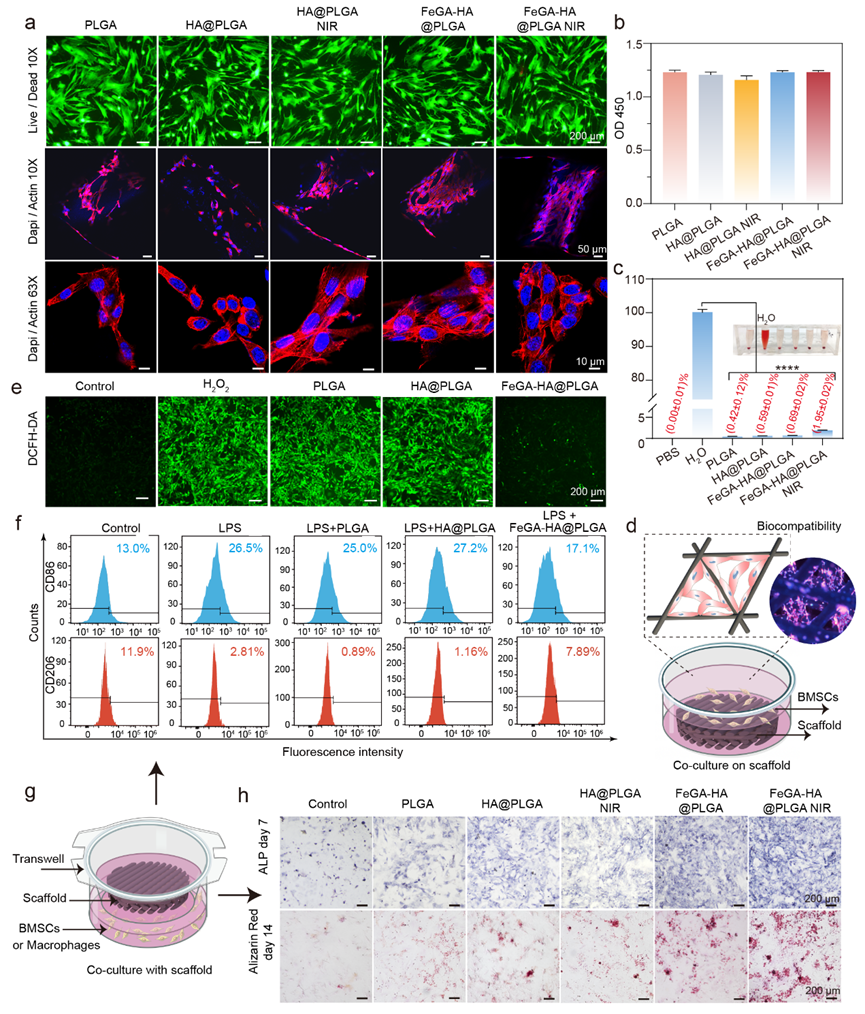
Figure 4. The biocompatibility, anti-inflammatory, and osteogenic differentiation of different scaffolds in BMSCs. (a) Live/Dead staining and cytoskeleton staining images (Dapi/Actin, confocal microscopy) of BMSCs cultured with different groups. Scale bar: 200 μm. (b) Cytotoxicity of the different scaffolds. (c) Hemolysis of rat blood treated with different scaffolds. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (d) Schematic diagram of cell-scaffold co-culture models for Live/Dead and Cytoskeleton staining. (e) The fluorescence images show intracellular ROS levels of BMSCs after being treated with different scaffolds. ROS was stained by DCF-DA. Scale bar: 200 μm. (f) Flow cytometry results showing CD206 and CD86 expression of macrophages with different treatments and represented as flow histograms. (g) Schematic diagram of cell-scaffold co-culture models for ROS staining. (h) ALP and ARS staining images of BMCSs co-cultured with different groups.
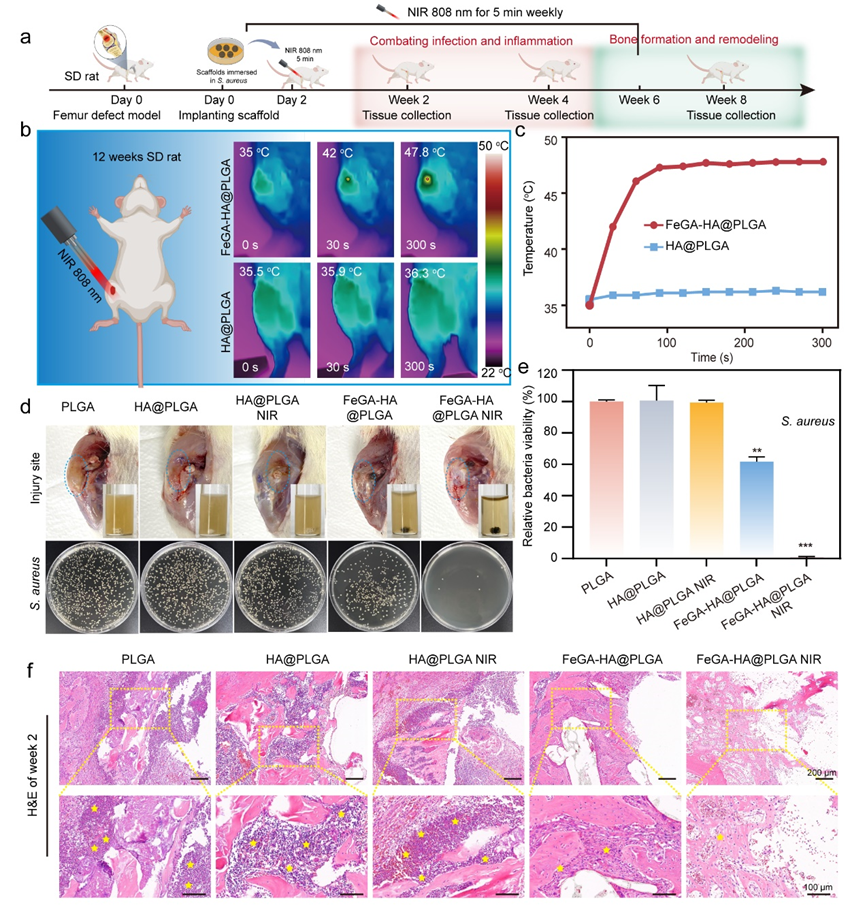
Figure 5. Characterization of in vivo antibacterial ability of FeGA-HA@PLGA scaffolds. (a) Schematic illustration of the animal experiment timeline. (b, c) Thermal graphics (b) and temperature changes (c) of implant sites for the HA@PLGA and FeGA-HA@PLGA groups under NIR irradiation (808 nm, 1.5 W/cm2). (d) Top: photographs of the implant sites and media cultured with the implanted scaffolds; Bottom: photographs of bacterial colonies co-cultured with different scaffolds. (e) In vivo antibacterial efficiency of different implanted scaffolds. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (f) The H&E staining images show the degree of infection in the soft tissues and bone tissues surrounding the implants (yellow stars represent the neutrophil cells).
综上所述,该研究通过结合仿生微结构和铁活性离子来调节铁稳态,为IBD的再生提供了一种前瞻性策略,这种铁活性多功能支架为感染性骨缺损修复应用提供了广泛的意义。
陈峰研究员为该研究论文的第一通讯作者,同济大学附属第十人民医院为第一通讯单位,赵新宇副研究员、王艳教授为共同通讯作者;同济大学2021级博士研究生尹梦婷、刘志清为本文的共同第一作者。本研究得到国家重点研发计划、国家自然科学基金面上项目、上海市科学技术委员会项目等资助。
原文链接:
https://onlinelibrary.wiley.com/doi/10.1002/advs.202407251


