课题设计思路:基于表观遗传的靶向治疗。美国在研的149项淋巴瘤基金关注这些热点(2024)
时间:2024-10-17 18:00:30 热度:37.1℃ 作者:网络
淋巴瘤(Lymphoma)是由乙型肝炎病毒(HBV)引起的一种传染性肝炎,可以导致急性或慢性肝病,慢性乙型肝炎可能导致肝硬化、肝衰竭甚至肝癌。该病毒通过血液、性接触或从母亲传给新生儿的途径传播。
尽管近年来在淋巴瘤的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
疫苗接种的挑战:虽然有高效的乙型肝炎疫苗,但全球仍有大量未接种疫苗的人群,尤其是在资源有限的国家。
-
治疗耐药性:现有的抗病毒药物虽然可以控制病毒复制和减少肝脏损伤,但耐药性的发展仍是一个重要问题。
-
慢性感染的管理:尽管抗病毒治疗可以有效控制病毒和延缓疾病进展,但目前还没有彻底治愈慢性乙型肝炎的方法。
-
筛查和诊断:在一些地区,由于缺乏资源,乙型肝炎的筛查和早期诊断仍然不足。
-
公共卫生教育和意识提升:提高公众对乙型肝炎传播途径和预防措施的认识是控制这种疾病扩散的关键。
解决这些问题需要全球卫生机构、政府和非政府组织的协作,以提高疫苗接种率,改进抗病毒治疗方案,加强公共健康教育,并推广筛查和早期诊断措施。
我们仅对美国国立卫生研究院(NIH)资助的在研淋巴瘤相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Lymphoma”为检索词、在题目中进行检索,美国NIH针对淋巴瘤的在研有149项。
一,谁获得了这些研究?
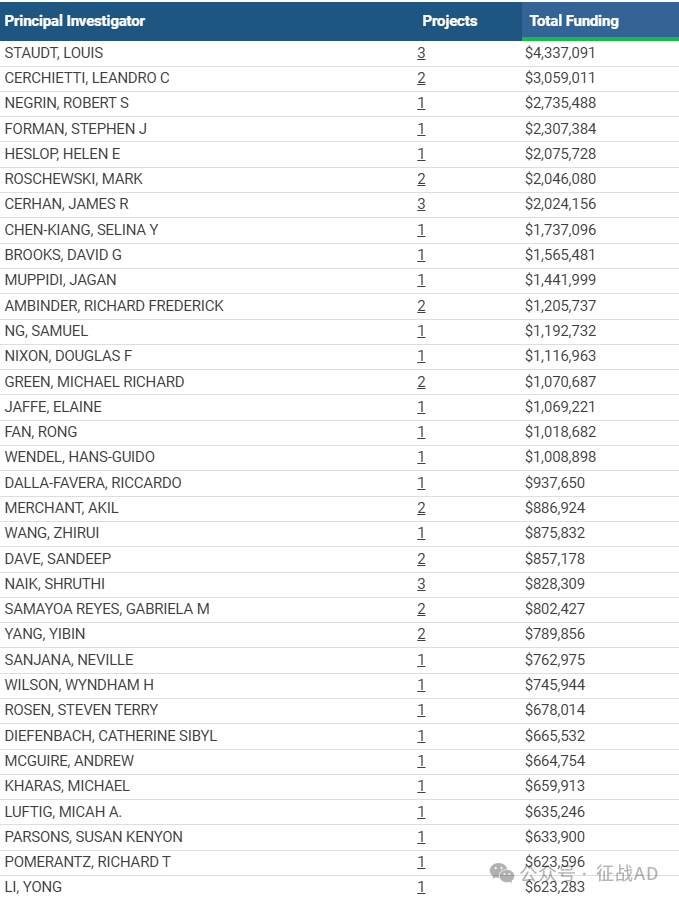
1,在研淋巴瘤基金最多的PI
-
DIVISION OF BASIC SCIENCES - NCI 的 STAUDT, LOUIS
-
WEILL MEDICAL COLL OF CORNELL UNIV 的 CERCHIETTI, LEANDRO C
-
STANFORD UNIVERSITY 的 NEGRIN, ROBERT S
-
BECKMAN RESEARCH INSTITUTE/CITY OF HOPE 的 FORMAN, STEPHEN J
-
BAYLOR COLLEGE OF MEDICINE 的 HESLOP, HELEN E
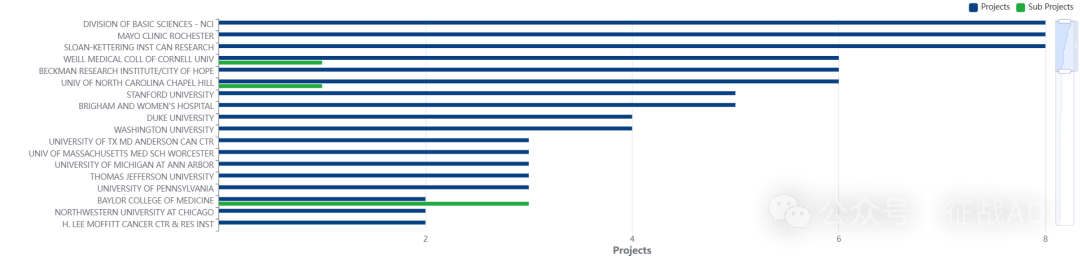
2,淋巴瘤基金最多的研究机构
-
基础科学部-NCI
-
康奈尔大学威尔医学院
-
贝克曼研究所/希望之城
-
斯坦福大学
-
罗切斯特梅奥诊所等
二,淋巴瘤研究热点是什么?
淋巴瘤研究领域总览(根据关键词)
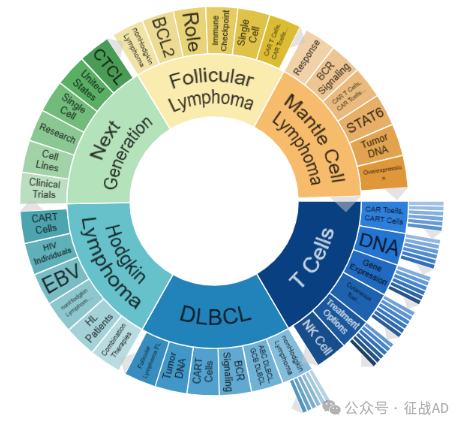
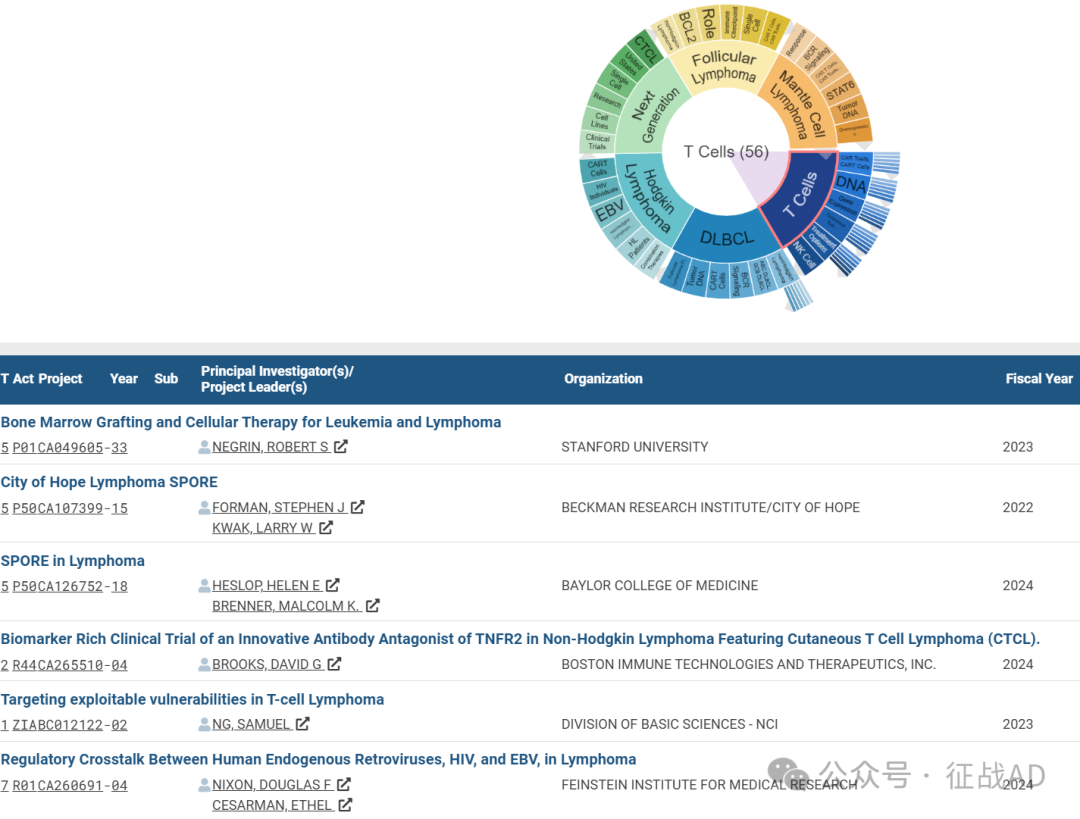
A,关于T细胞(T Cells)的研究项目最多
有 56 项在研基金涉及到了T细胞,关注最多的方面包括CAR T细胞(CAR T Cells)、DNA、基因表达(Gene Expression)、皮肤T细胞淋巴瘤(Cutaneous T Cell Lymphoma)、治疗方案(Treatment Options)、NK细胞(NK Cell)等研究。
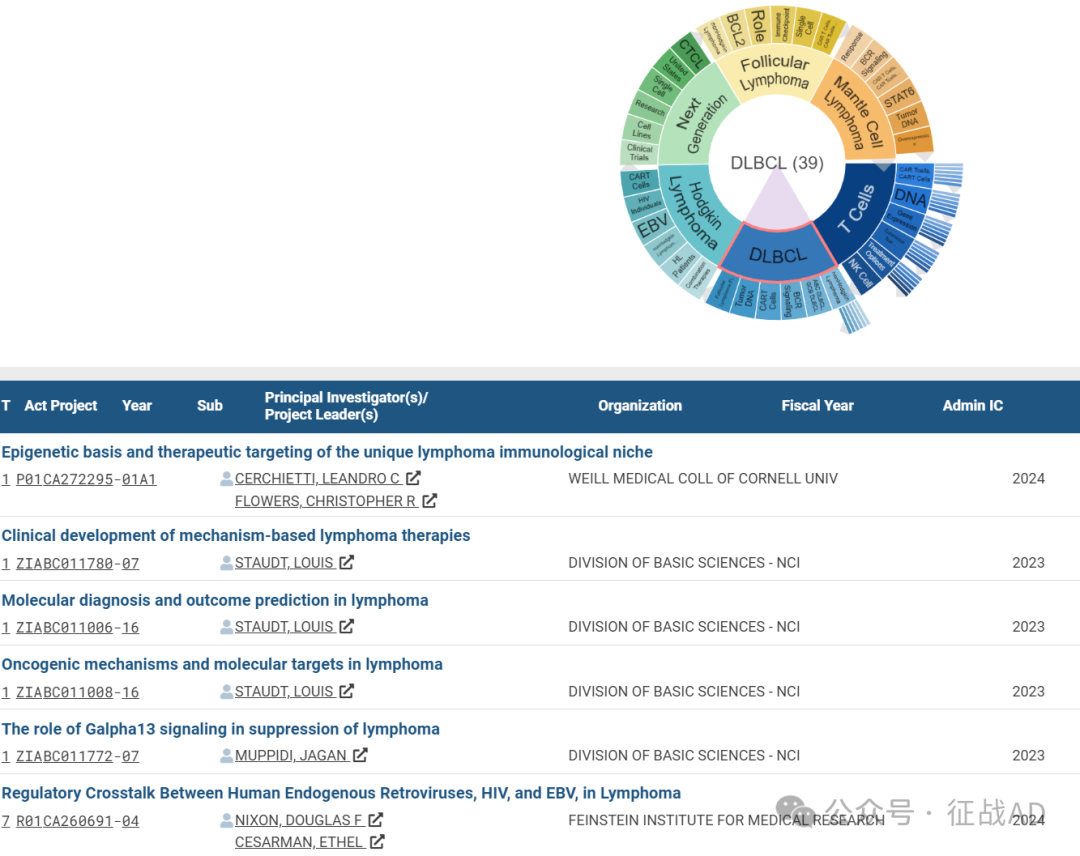
B,关于弥漫大B细胞淋巴瘤(DLBCL)的研究项目
有 39 项在研基金涉及到了弥漫大B细胞淋巴瘤,关注最多的方面包括非霍奇金淋巴瘤(nonHodgkin Lymphoma)、ABC DLBCL、GCB DLBCL、BCR 信号传导(BCR Signaling)、CAR T细胞(CAR T Cells)、肿瘤DNA(Tumor DNA)、滤泡性淋巴瘤(Follicular Lymphoma, FL)等研究。
C,关于霍奇金淋巴瘤(Hodgkin Lymphoma)的研究项目
有 22 项在研基金涉及到了霍奇金淋巴瘤,关注最多的方面包括联合疗法(Combination Thrapies)、HL 患者(HL Patients)、非霍奇金淋巴瘤(nonHodgkin Lymphoma)、EBV、HIV 患者(HIV Individuals)、CAR T细胞(CAR T Cells)等研究。
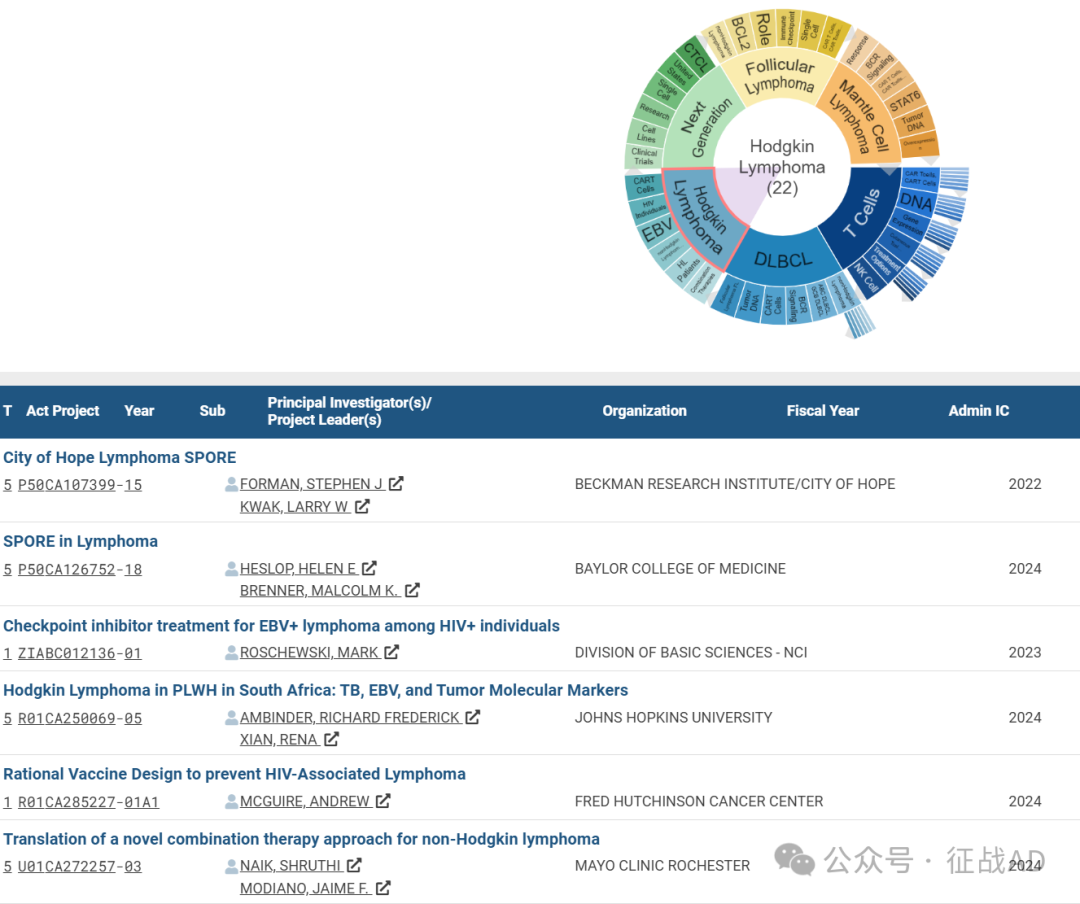
其他淋巴瘤研究大的方向还包括下一代(Next Generation)、滤泡性淋巴瘤(Follicular Lymphoma)、套细胞淋巴瘤(Mantle Cell Lymphoma)等。
三,借鉴与突破
我们也分享在淋巴瘤领域的几项课题摘要,希望对同仁们有所启发。
A,Bone Marrow Grafting and Cellular Therapy for Leukemia and Lymphoma
The overall goals of our Program Project Grant are to develop a more robust fundamental understanding of transplantation biology and address the major challenges of HCT including GVHD, disease relapse and immune reconstitution. We will tackle these major challenges through innovative animal modeling, comprehensive biological and molecular analysis, novel imaging of animal and human subjects and biologically focused translational clinical trials. In addition, we will explore the biology of key cellular populations including Treg, iNKT, TR1, CD8 memory and CAR T cells.
Our Program involves 5 interactive Projects focusing on the biology and prevention of GVHD through an enhanced understanding of cellular imaging and immune regulation (Projects 1 and 2), prevention and treatment of disease relapse with memory CD8+ T cells and vaccination strategies (Project 3), improved function and efficacy of CAR T cells (Project 4) and monoclonal antibody based strategies (Project 5).
These Projects are supported by three Cores (Administration and Clinical Trial Coordination, Biostatistics and Data Management and Cell Processing and Immune Monitoring).
Through our interactive Program Project, we will gain novel insights into the biology of hematopoietic cell transplantation and develop innovative strategies to improve outcomes for patients with hematological malignancies. The knowledge gained has profound implications for cancer and transplantation biology and also for the treatment of patients with a broad range of immunological conditions such as autoimmune disorders and transplantation of solid organs.
B, Epigenetic basis and therapeutic targeting of the unique lymphoma immunological niche
To address this challenge we have assembled a team of experts with distinct spheres of expertise, suites of cutting edge technologies and powerful and physiologically accurate model systems. They have worked together as a team to develop a compelling body of preliminary data supporting the conceptual framework of this proposal, and have developed critical resources such as shared genomics databases, primaryhuman PDX repositories, genetically engineered mouse models, computational pipelines and graphical user interfaces, and others.
They will address the central hypotheses that i) chromatin is the key integrator, rheostat and executor of cell-intrinsic and cell- extrinsic pathways regulating key GC cell fate decisions downstream of immune synapse signaling, ii) DLBCLs reflect a continuum of malignant states with each tumor aligning with one or more points along GC B-cell epigenetic trajectories, iii) GCB and ABC DLBCLs are defined by crosstalk between their chromatin landscapes and other immune populations to shape the LME, iv) that these chromatin to LME effects vary in specific ways with mutation profiles and with aging age, and that v) rational targeting of DLBCLs must take into account and specifically antagonize these specific chromatin-immune synapse - LME scenarios to achieve long term tumor eradication.

